TIM-3 therapy for Alzheimer’s emerges as a groundbreaking approach in the fight against this debilitating disease, offering new hope for effective Alzheimer’s treatment. A recent study has shown that inhibiting the TIM-3 checkpoint molecule can revitalize the brain’s immune cells, known as microglia, enabling them to effectively combat Alzheimer’s plaques and restore memory function in animal models. By turning off this inhibitory pathway, researchers have demonstrated improved cognitive abilities, paving the way for potential applications in human patients. This innovative strategy not only sheds light on the role of the immune system in Alzheimer’s but also draws parallels with successful cancer therapies that utilize similar mechanisms. As research progresses, TIM-3 therapy could redefine how we understand and treat Alzheimer’s disease, potentially transforming the lives of millions affected by cognitive decline.
New advancements in Alzheimer’s therapy, particularly the TIM-3 strategy, represent a shift in how we perceive immune interactions within the brain. Known for its role in regulating immune responses, TIM-3 could be key to unlocking effective treatments for Alzheimer’s disease. By targeting this checkpoint molecule, we can enhance the brain’s innate immune response, allowing microglia to clear harmful amyloid plaques that hinder cognitive function. The intersection of cancer research and neurodegenerative disease treatments paves an exciting avenue to explore immune system modulation as a viable option in addressing conditions like Alzheimer’s. As we delve deeper into the implications of TIM-3 modulation, the potential for innovative solutions in Alzheimer’s management becomes increasingly promising.
The Role of TIM-3 in Alzheimer’s Disease
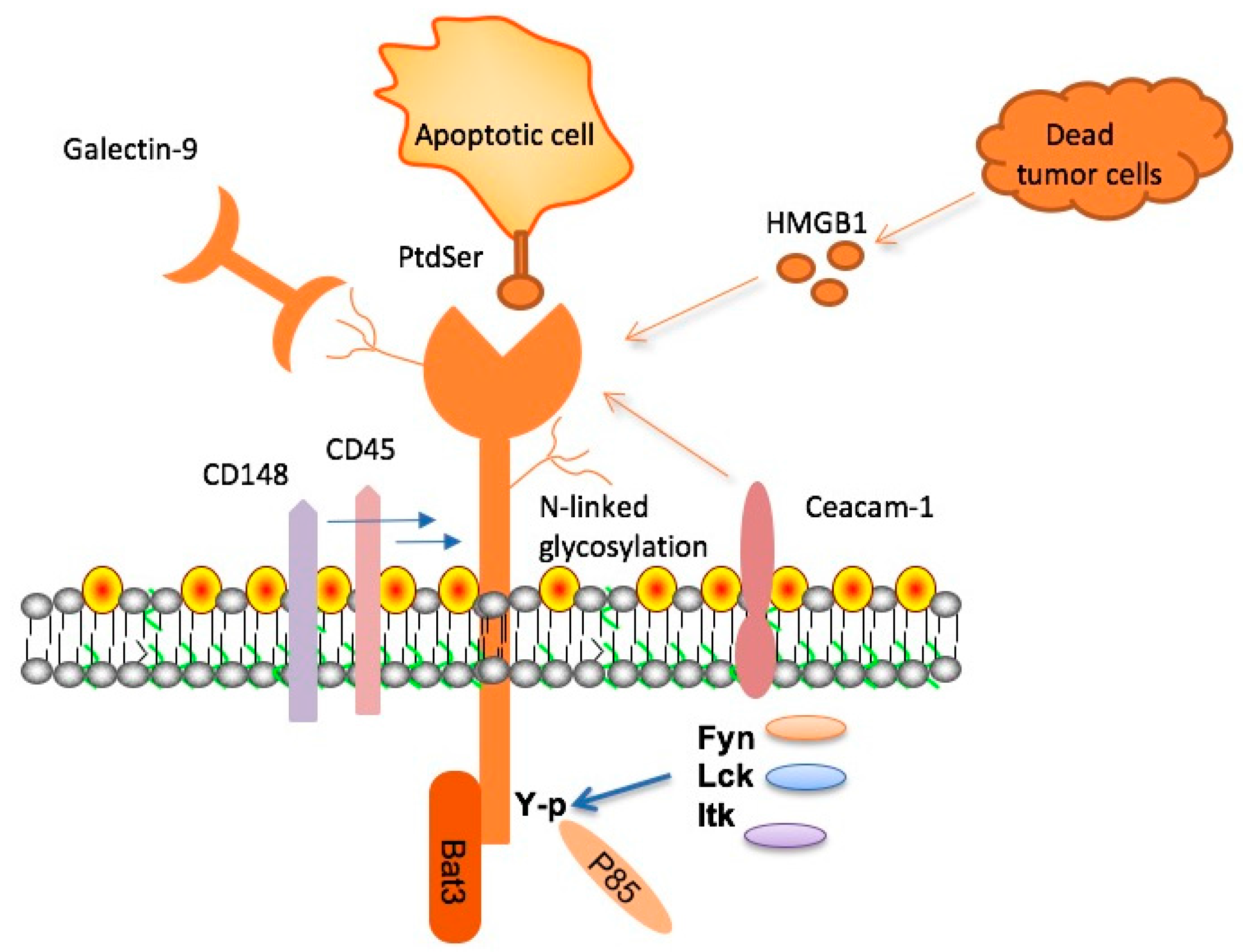
TIM-3, or T-cell immunoglobulin mucin-3, is an inhibitory molecule that plays a crucial role in the immune system’s regulation. In the context of Alzheimer’s disease (AD), TIM-3 has been identified as a significant factor that hinders microglia, the brain’s immune cells, from efficiently clearing harmful amyloid plaques. These plaques contribute to cognitive decline and memory loss in individuals with Alzheimer’s. The connection between TIM-3 and late-onset Alzheimer’s disease suggests it could serve as a critical target for therapeutic interventions aimed at enhancing immune responses against these damaging proteins in the brain.
In lab studies, when TIM-3 expression was inhibited in genetically modified mice, researchers observed remarkable changes in plaque management. Microglia were able to engage and clear amyloid plaques more effectively, which not only reduced plaque size but also improved cognitive functions in these animals. This finding highlights the potential of TIM-3-targeted therapies as a groundbreaking approach to treating Alzheimer’s disease by leveraging the body’s immune system to combat the condition.
TIM-3 Therapy: A Potential Breakthrough for Alzheimer’s Treatment
Research into TIM-3 therapy for Alzheimer’s shows promising potential as it borrows from successful cancer treatment strategies. By blocking the inhibitory effects of TIM-3 on microglia, this therapy aims to restore the immune system’s ability to clear amyloid plaques from the brain. Anti-TIM-3 antibodies or small molecules could be administered to Alzheimer’s patients to activate microglia effectively, allowing them to respond to and eliminate plaques that contribute to the disease’s progression.
This concept builds on the understanding that inhibiting checkpoint molecules like TIM-3 has already yielded benefits in cancer immunotherapy. If successfully translated to Alzheimer’s treatment, TIM-3 therapy could halt or even reverse the cognitive decline associated with the disease. Early studies indicate that such therapies could be less likely to lead to vascular damage compared to existing anti-amyloid treatments, as TIM-3 selectively targets the microglial response rather than circulating antibodies affecting brain endothelial cells.
Understanding Microglia: The Immune Cells of the Brain
Microglia serve as the brain’s primary immune cells, constantly on alert to maintain a healthy environment by pruning synapses and clearing debris. As individuals age, however, the function of microglia can diminish, with increased TIM-3 expression causing them to become homeostatic. This shift leads to reduced efficacy in clearing amyloid beta plaques and can contribute to the development of Alzheimer’s disease. Understanding the role of microglia in synaptic health and their response to parental aging is essential for identifying potential therapeutic targets for AD.
In Alzheimer’s patients, it’s observed that microglia express significantly higher levels of TIM-3, reflecting a dysfunctional state where they fail to actively engage with and eliminate pathological amyloid plaques. This impairment suggests that interventions aimed at decreasing TIM-3 expression could restore the natural phagocytic function of microglia, allowing them to clear plaques and potentially alleviate memory impairments associated with the disease.
The Genetic Link Between TIM-3 and Late-Onset Alzheimer’s
The connection between TIM-3 and late-onset Alzheimer’s disease has been substantiated by genome-wide association studies that identify TIM-3, or HAVCR2, as a genetic risk factor. This polymorphism indicates that individuals with specific variations in the TIM-3 gene may exhibit a heightened susceptibility to developing Alzheimer’s as they age. The molecular mechanisms behind this link reveal that TIM-3’s role as a checkpoint molecule not only affects immune responses but is also critical in maintaining cognitive health.
Moreover, the presence of TIM-3 on microglia in Alzheimer’s patients provides a potential target for new treatments. By focusing on individuals with this specific genetic predisposition, scientists can tailor therapies that counteract the harmful effects of TIM-3, thereby enhancing the brain’s innate ability to clear harmful plaques and protect cognitive function in the aging population.
Current Research and Future Perspectives on TIM-3 Therapy
Recent studies exploring TIM-3 therapy for Alzheimer’s disease highlight the need for innovative approaches after several unsuccessful drug trials in the past. Research teams, including those from Harvard Medical School, are investigating the use of TIM-3 inhibitors to enhance microglial activity in Alzheimer’s mouse models. By observing the behavioral improvements in these models, researchers aim to gather compelling evidence of the potential benefits of targeting TIM-3 in human subjects with Alzheimer’s.
The future of TIM-3 therapy involves using genetically modified mouse models that incorporate the human TIM-3 gene, leading to more accurate preclinical testing for therapies available to humans. As exciting discoveries emerge, there may be pathways leading to the development of new, effective treatments that harness the body’s immune system, paving the way for enhanced management of Alzheimer’s disease and a comprehensive strategy to combat its debilitating effects on memory and cognition.
Challenges in Alzheimer’s Disease Treatment and TIM-3 Potentials
Despite progress made in Alzheimer’s research, numerous challenges remain in developing effective therapies. Conventional anti-amyloid treatments have shown limited success, often failing to demonstrate significant cognitive benefits while presenting risks of vascular complications. This positions TIM-3 therapy as a unique opportunity to re-evaluate treatment strategies, emphasizing the importance of harnessing the immune system’s potential without the associated side effects of existing treatments.
The versatility of TIM-3-targeting strategies could provide a much-needed alternative for Alzheimer’s treatment. By focusing on the disease’s underlying biology and the role of microglia, there’s hope that TIM-3 therapy can not only mitigate plaque accumulation but also enhance cognitive function significantly, transforming the landscape of Alzheimer’s disease management.
The Mechanism of Immune System Activation in Alzheimer’s Therapy
One of the most exciting aspects of TIM-3 therapy is its mechanism of action. By disabling the inhibitory signals sent by TIM-3, microglia are empowered to engage actively with amyloid plaques, promoting their clearance and allowing cognitive functions to recover. This shift represents a significant departure from traditional models of treatment that focus solely on reducing plaque burden, showcasing a holistic approach that involves reinstating the immune system’s natural capabilities.
Activating the immune system through TIM-3 inhibition not only targets plaque-related pathology but also aims to rejuvenate the overall health of the brain. This dual benefit could help slow the progression of Alzheimer’s disease while concurrently restoring some degree of normal memory function, an appealing prospect for both patients and researchers alike.
Collaborative Efforts in Alzheimer’s Research and TIM-3 Development
The multidisciplinary approach to Alzheimer’s research is essential for rapidly transitioning discoveries into clinical applications. The collaboration between experts in neurology, immunology, and genetics underscores the importance of diverse perspectives in the exploration of TIM-3 therapy for Alzheimer’s. Combining expertise allows for an innovative framework that addresses the multifaceted nature of Alzheimer’s disease and effectively targets both immune dysfunction and pathogenic processes.
Involving institutions, researchers, and funding bodies further enhances the pace at which advancements can be achieved. Shared resources and knowledge enable quicker analysis of TIM-3’s role in Alzheimer’s, optimizing the development and testing of potential therapies. Through collective effort and dedication, the outlook for combating Alzheimer’s disease through TIM-3 therapy can become a reality.
Alzheimer’s Treatment Landscape: Integrating TIM-3 Insights
The landscape of Alzheimer’s treatment continues to evolve with insights gained from TIM-3 studies alongside other therapeutic avenues. By synthesizing findings from immune system research and traditional Alzheimer’s treatments, a more comprehensive strategy can emerge to offer holistic solutions for patients battling this complex disease. TIM-3 therapy presents an innovative angle that could realign existing treatment paradigms, emphasizing the immune response as a critical component of effective therapy.
Incorporating TIM-3 findings with ongoing research in other areas of Alzheimer’s treatment, such as the neuroinflammatory response and tau pathology, could pave the way for combination approaches that enhance patient outcomes. Ultimately, integrating TIM-3 insights with broader therapeutic strategies holds the potential for developing more effective, targeted treatments that may significantly improve the quality of life for those affected by Alzheimer’s disease.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s leverages the inhibition of the TIM-3 checkpoint molecule, which restricts the activity of microglia—immune cells in the brain. Recent studies show that blocking TIM-3 allows microglia to clear amyloid plaques, leading to improved cognitive function. This approach transforms the TIM-3 pathway, which is typically exploited in cancer therapy, to enhance the immune response against Alzheimer’s plaques.
How is TIM-3 related to the immune system in Alzheimer’s treatment?
TIM-3 is a checkpoint protein that regulates immune responses, including those in Alzheimer’s disease. It inhibits microglia from attacking harmful amyloid plaques in the brain, leading to cognitive decline. By targeting TIM-3, therapeutic strategies aim to boost microglial activity, allowing these immune cells to effectively clear plaques and potentially restore memory functions.
Can TIM-3 therapy for Alzheimer’s benefit those with late-onset Alzheimer’s?
Yes, TIM-3 therapy holds promise for treating late-onset Alzheimer’s, which accounts for the majority of cases. Research indicates a genetic link between TIM-3 expression and late-onset Alzheimer’s, suggesting that therapies targeting this checkpoint molecule may help alleviate symptoms by enhancing microglial plaque clearance.
What are the potential side effects of TIM-3 therapy for Alzheimer’s patients?
While TIM-3 therapy for Alzheimer’s is still in experimental stages, potential side effects may include alterations in immune function due to the modulation of microglial behavior. Close monitoring will be necessary to ensure that enhancing microglial activity does not lead to unintended consequences, such as increased inflammation.
How does the deletion of TIM-3 gene in mice demonstrate the therapy’s effectiveness?
In studies where the TIM-3 gene was deleted in mice, researchers found that microglia could effectively remove amyloid plaques from the brain. This therapeutic model shows improved memory and cognitive function, highlighting that removing the inhibitory effects of TIM-3 can significantly benefit Alzheimer’s treatment and provide insights for human applications.
What research supports the use of TIM-3 therapy in Alzheimer’s plaques treatment?
Recent research published in high-impact journals has demonstrated that TIM-3 plays a critical role in inhibiting microglial clearance of amyloid plaques. By inhibiting TIM-3 in mouse models of Alzheimer’s, researchers observed cognitive improvement and better plaque management, paving the way for future clinical applications of TIM-3 therapy in Alzheimer’s treatment.
Is TIM-3 therapy being tested in human trials for Alzheimer’s?
Currently, TIM-3 therapy is in the preclinical stage, focusing on mouse models. However, the transition to human trials is a goal, as researchers are modifying models to test anti-TIM-3 antibodies for their potential efficacy in halting plaque development in patients with Alzheimer’s. Future studies will be crucial to validate these findings in humans.
| Key Points |
|---|
| Research indicates that TIM-3 therapy may improve cognitive function in Alzheimer’s patients by enabling microglia to attack amyloid plaques. |
| TIM-3 is an immune checkpoint molecule that inhibits microglia from clearing plaques. |
| Genetic polymorphisms in the TIM-3 gene increase the risk of late-onset Alzheimer’s disease. |
| The study was a collaboration involving multiple researchers over a five-year period, showing significant progress in Alzheimer’s therapy. |
| Removing TIM-3 expression in mice led to improved memory and reduced plaque accumulation in the brain. |
| Future treatments may involve the use of antibodies or small molecules to inhibit TIM-3 activity in humans. |
Summary
TIM-3 therapy for Alzheimer’s presents a promising avenue for treating this devastating disease. Recent research highlights the role of TIM-3, a checkpoint molecule that inhibits the brain’s immune cells, preventing them from clearing harmful amyloid plaques. By deleting TIM-3, studies in mouse models have shown improvements in memory and cognitive function. This innovative approach, which repurposes existing TIM-3 antibodies, offers hope in the fight against Alzheimer’s disease and could lead to significant advancements in therapeutic strategies.