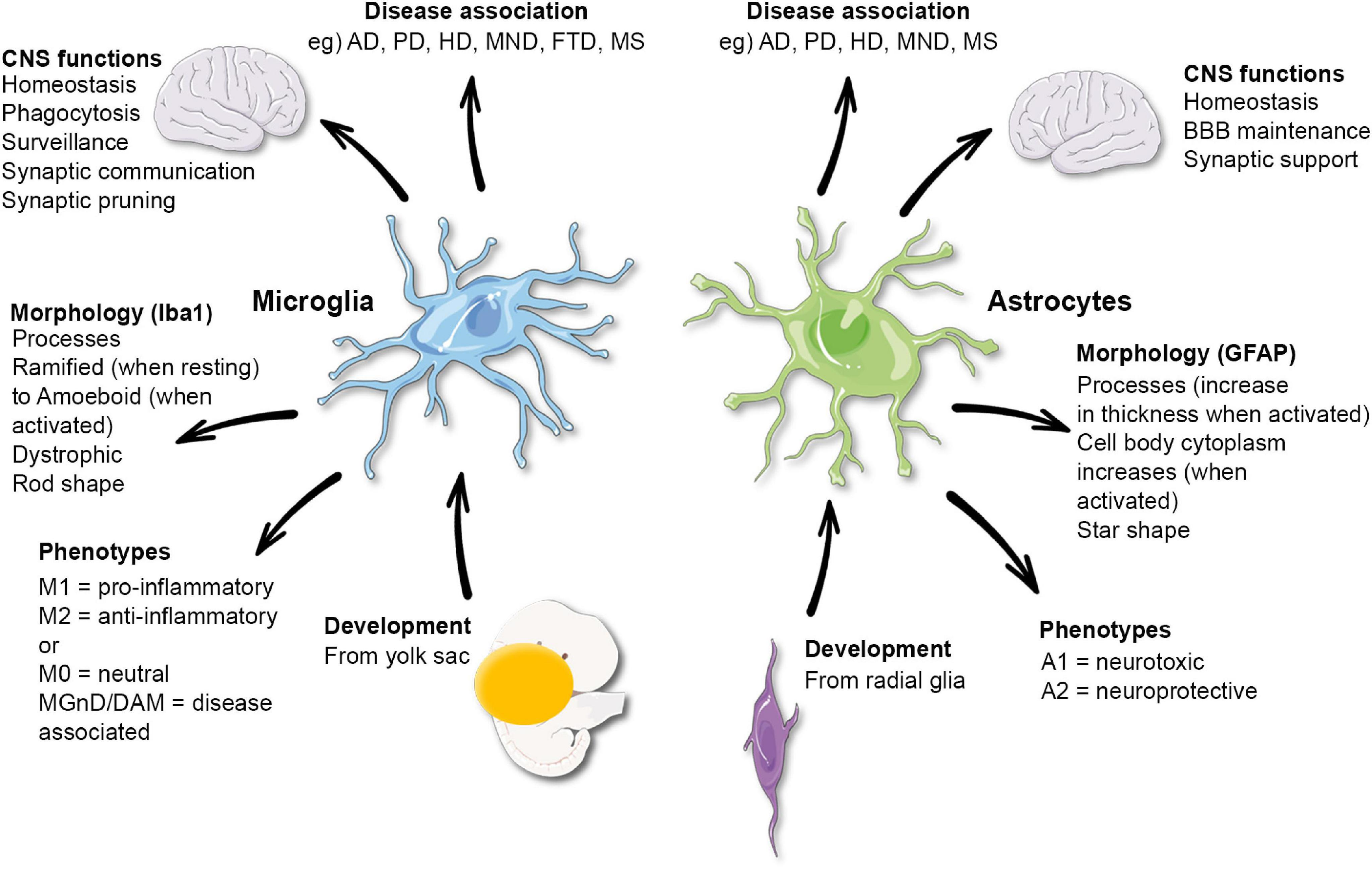
Microglial research is transforming our understanding of the brain’s immune system, particularly in relation to neurodegenerative diseases like Alzheimer’s disease. Scientists, including Beth Stevens, are uncovering how microglia, the brain’s primary immune cells, actively monitor and maintain neuronal health. These cells play a crucial role in clearing out dead or damaged neurons and synaptic pruning, processes vital for brain function. However, when microglial functions go awry, they can inadvertently contribute to disorders like Alzheimer’s and Huntington’s disease. As research continues to reveal the intricate workings of microglia, it holds promise for developing biomarkers and treatments that could significantly impact millions suffering from these debilitating conditions.
Research into microglial cells is shedding light on the complexities of the brain’s defense mechanisms, offering fresh perspectives on how they relate to conditions such as Alzheimer’s disease. These glial cells act as custodians, ensuring proper communication among neurons by managing the clearance of debris and the refinement of synapses. Insights from leading researchers like Beth Stevens highlight the dual role of microglia; they are vital for maintaining cognitive health yet can contribute to neurodegenerative diseases when their pruning activities become dysfunctional. This growing understanding of the relationship between microglial behavior and neurological disorders is pivotal in paving the way for innovative therapeutic strategies targeting the brain’s unique immune landscape. As studies deepen, the potential to translate these findings into effective treatments grows, promising new hope for those impacted by neurodegeneration.
The Role of Microglial Research in Alzheimer’s Disease
Microglial research has emerged as a groundbreaking field in understanding the complexities of Alzheimer’s disease. Microglia, the innate immune cells of the brain, play a pivotal role in maintaining neurological health by constantly surveilling for signs of injury or illness. Recent discoveries led by Beth Stevens have illuminated the dual role of microglia; while these cells are essential for clearing debris and supporting synaptic connections, they can also contribute to neurodegeneration if their pruning processes go awry. This aberrant pruning has been linked to the pathology of Alzheimer’s disease, demonstrating that maintaining the balance of microglial activity is crucial for cognitive health.
In Stevens’ research at the Boston Children’s Hospital, her team has focused on uncovering the mechanisms by which microglia operate within the brain’s immune system. By advancing our understanding of how microglial cells interact with neurons and contribute to disease, Stevens’ work paves the way for new therapeutic approaches that target these immune cells. This has significant implications not only for Alzheimer’s disease treatment but also for designing interventions for other neurodegenerative diseases, reinforcing the importance of microglial research as a cornerstone of modern neuroscience.
Understanding Neurodegenerative Diseases Through Microglial Activity
Neurodegenerative diseases like Alzheimer’s and Huntington’s result from a complex interplay of genetic, environmental, and cellular factors. A critical aspect of this interplay involves the brain’s immune response, predominantly mediated by microglia. These cells are pivotal in modulating neuroinflammation, which can have protective or detrimental effects on brain health. The insights gained from microglial research are shedding light on how these cells can either safeguard the brain’s integrity or contribute to its decline when their functioning is disrupted.
By leveraging models of neurodegenerative diseases, researchers are beginning to characterize the pathogenic roles that dysfunctional microglia play. Stevens’ innovative approach involves understanding the genetic and environmental triggers that lead to altered microglial behavior. This research is essential for identifying biomarkers that could signal the onset of neurodegeneration, ultimately guiding early interventions. As scientists continue to unravel the complexities of neurodegenerative diseases through microglial activity, the potential for new, targeted therapies becomes increasingly exciting.
Advancements in Brain Immune System Research
The investigation of the brain immune system, particularly the role of microglia, has gained significant traction in recent years. Beth Stevens emphasizes the transformation in our understanding of microglial function in health and disease. These cells were long thought to be merely support cells in the brain, but current studies reveal their active participation in synaptic pruning and overall neurodevelopment. This pioneering perspective expands our knowledge of how microglia respond to various neurological conditions, emphasizing their involvement in both neuroprotection and neurodegeneration.
Stevens’ work underscores the importance of federal research funding in propelling these scientific advancements. The ability to explore the intricacies of the brain’s immune system, driven by curiosity and a commitment to basic science, has led to groundbreaking findings about microglia’s roles. As researchers continue to delve into the relationships between immune responses and neurodegenerative disorders, the foundational science that supports this exploration becomes increasingly vital for addressing the challenges posed by diseases like Alzheimer’s.
Discovering New Biomarkers for Alzheimer’s Disease
The quest for reliable biomarkers in Alzheimer’s disease has gained momentum through the lens of microglial research. By analyzing the behaviors and profiles of microglial cells in various states, scientists like Beth Stevens are uncovering potential indicators of neurodegeneration that could lead to earlier diagnosis and treatment. Biomarkers derived from microglial function may provide critical insights into the progression of Alzheimer’s, allowing for interventions to be implemented before significant cognitive decline occurs.
Stevens’ findings point to unique patterns of microglial activation that correlate with the presence of amyloid plaques, a hallmark of Alzheimer’s disease. By integrating these discoveries with existing diagnostic processes, researchers aim to refine the methods used to predict Alzheimer’s onset in at-risk populations. The implications of identifying such biomarkers are profound, potentially transforming clinical practice and enabling a more proactive approach to managing neurodegenerative diseases.
The Impact of Curiosity-Driven Science on Alzheimer’s Research
Curiosity-driven science is vital in advancing our understanding of complex neurological conditions, such as Alzheimer’s disease. Beth Stevens exemplifies this approach, highlighting how initial explorations into the brain’s immune system have led to significant breakthroughs in microglial research. Her determination to follow scientific inquiry without a predetermined outcome has resulted in pivotal insights into the mechanisms underlying synaptic pruning and its implications in neurodegeneration. This emphasizes the necessity of funding and support for basic research, which often leads to unexpected yet valuable discoveries.
The dynamic nature of scientific research means that each discovery opens up new avenues for inquiry. For example, Stevens’ foundational work on microglia has not only broadened the understanding of their role in neuroinflammation but has also sparked interests in potential therapeutic interventions. This highlights the importance of maintaining curiosity in scientific pursuits, as it allows for the exploration of uncharted areas that may yield crucial insights into the treatment of Alzheimer’s and other neurodegenerative conditions.
Future Directions in Neuroimmune Research
As we move forward, the integration of neuroimmune research with neurodegenerative disease studies presents exciting possibilities. Beth Stevens and others in the field stress the importance of understanding how the brain’s immune cells can be harnessed to combat diseases like Alzheimer’s. New therapeutic strategies that modulate microglial activity to enhance brain health are on the horizon, driven by ongoing research that reveals the complex interactions between immune responses and neuronal function.
The future of neuroimmune research holds promise for not only improving our understanding of Alzheimer’s disease but also for addressing a wider range of neurodegenerative disorders. By focusing on the multifaceted roles of microglia, researchers are poised to develop innovative treatments that could mitigate the devastating effects of these diseases, ultimately improving the lives of millions affected by Alzheimer’s worldwide.
Funding the Future of Alzheimer’s Research
Adequate funding is crucial for the progress of Alzheimer’s research, particularly in the realm of microglial studies. The insights derived from the NIH-supported research conducted by Beth Stevens and her team exemplify how federal funding can catalyze transformative discoveries in neurobiology. By securing grants and resources, researchers are empowered to explore complex questions about the brain’s immune system which, in turn, yields findings that could lead to novel therapeutic strategies for Alzheimer’s and other neurodegenerative diseases.
Continued investment in Alzheimer’s research is vital, especially as the number of patients rises. With 7 million Americans currently affected by Alzheimer’s, it is imperative to support initiatives that enhance our understanding of the disease. Through sustained funding for curiosity-driven research, scientists can delve deeper into the roles of microglia and neuroinflammation, potentially leading to breakthroughs that improve diagnostic frameworks and treatment paradigms for those impacted by this devastating condition.
The Importance of Basic Science in Alzheimer’s Research
The importance of basic science in understanding Alzheimer’s disease cannot be overstated. Beth Stevens emphasizes that many of the breakthroughs in microglial research have stemmed from foundational studies, often unfocused on immediate clinical applications. This approach to science allows researchers to explore fundamental biological questions that are crucial for unraveling the complexities of neurodegenerative diseases. Basic science serves as the bedrock upon which applied research is built, ensuring that discoveries are both robust and scientifically rigorous.
Understanding the underpinning mechanisms of microglial function and their impact on synaptic health highlights the necessity of basic research within the field of neuroscience. As Beth Stevens has shown, these exploratory studies may not only enhance our knowledge but can also lead to innovative treatments for Alzheimer’s disease. By prioritizing curiosity and foundational science, the path forward for Alzheimer’s research becomes clearer, paving the way for advances that could ultimately change the way we approach neurodegeneration.
The Multidimensional Approach to Alzheimer’s Care
Alzheimer’s care requires a multifaceted approach that considers the interplay of neurobiology, social factors, and patient needs. Stevens’ research highlights the importance of understanding microglial processes in the development of holistic care strategies. By acknowledging the role of the brain’s immune response in neurodegeneration, caregivers and healthcare professionals can better support patients and their families. This multidimensional perspective can lead to improved therapeutic outcomes and enhanced quality of life for those living with Alzheimer’s disease.
Integrating microglial research into personalized Alzheimer’s care plans offers an opportunity to tailor interventions to individual patients based on their unique biological profiles. By leveraging the insights gained from studies on microglia and neuroinflammation, caregivers can approach Alzheimer’s treatment with a more nuanced understanding of disease mechanisms. This alignment of research with clinical practices not only fosters improved care but also reinforces the importance of ongoing research in informing and innovating patient care strategies.
Frequently Asked Questions
What role do microglia play in Alzheimer’s disease research?
Microglia are essential components of the brain’s immune system and play a critical role in Alzheimer’s disease research. They are responsible for monitoring the brain for damage, clearing dead cells, and pruning synapses. Aberrant pruning by microglia has been linked to the progression of Alzheimer’s disease, making them a focal point for developing new biomarkers and therapeutic interventions.
How has Beth Stevens contributed to our understanding of microglial cells in neurodegenerative diseases?
Beth Stevens has significantly advanced our understanding of microglial cells in neurodegenerative diseases through her research. Her work at the Stevens Lab has revealed how microglia mismanage synaptic pruning, contributing to disorders like Alzheimer’s and Huntington’s disease. This foundational research is paving the way for new treatments and strategies to address these challenging diseases.
Why are microglial cells important for the brain’s immune system?
Microglial cells act as the brain’s immune system, protecting against injury and disease. They detect and respond to changes in the brain environment by clearing debris and regulating neuronal connections. Understanding their function is crucial in microglial research, especially in the context of neurodegenerative diseases such as Alzheimer’s, where their activity can become dysregulated.
What are the implications of microglial research for treating Alzheimer’s disease?
Microglial research has profound implications for treating Alzheimer’s disease. By understanding how microglia contribute to the disease process, researchers can develop targeted therapies that aim to correct aberrant microglial function, ultimately helping to prevent or mitigate the symptoms of Alzheimer’s in the millions affected.
How does aberrant pruning by microglia contribute to neurodegenerative diseases?
Aberrant pruning by microglia can lead to the loss of essential synaptic connections in the brain, which is seen in neurodegenerative diseases like Alzheimer’s. This mismanagement contributes to cognitive decline and other symptoms. Research is focused on deciphering these mechanisms to find ways to restore normal function and protect brain health.
What discoveries related to microglia have emerged from the Stevens Lab?
The Stevens Lab has made groundbreaking discoveries regarding the role of microglia in synaptic pruning and their impact on neurodegenerative diseases such as Alzheimer’s. These findings suggest new pathways for creating biomarkers and treatments that could change the approach to these diseases, influencing future care strategies for affected individuals.
How does microglial research align with advancements in treating neurodegenerative diseases?
Microglial research aligns with advancements in treating neurodegenerative diseases by uncovering mechanisms underlying synaptic loss and inflammation in conditions like Alzheimer’s. This research leads to the development of innovative therapeutic strategies aimed at restoring healthy microglial function, ultimately enhancing the potential for effective treatment.
Can understanding microglia lead to new biomarkers for Alzheimer’s disease?
Yes, understanding the role of microglia in Alzheimer’s disease can lead to the identification of new biomarkers. By studying how microglia react and change in response to pathological processes associated with Alzheimer’s, researchers can develop indicators that help in early diagnosis and monitoring disease progression.
| Key Points | Details |
|---|---|
| Role of Microglia | Microglia serve as the brain’s immune system, patrolling for illnesses and clearing damaged cells. |
| Impact on Neurodegenerative Diseases | Aberrant microglial pruning is linked to diseases like Alzheimer’s and Huntington’s. |
| Innovations from Research | Research by Beth Stevens has led to new biomarkers and potential treatments for Alzheimer’s. |
| Importance of Curiosity-Driven Science | Basic science is crucial for discovering new insights that translate into disease understanding and treatment. |
| Federal Funding Support | Stevens emphasizes the importance of NIH and federal support for advancing her research. |
Summary
Microglial research is crucial for understanding and combating neurodegenerative diseases like Alzheimer’s. Beth Stevens’ groundbreaking work highlights the essential role of microglia in the brain’s immune response and their impact on synaptic health. By investigating the dynamics of microglial behavior and its implications on diseases, Stevens is paving the way for innovative treatment strategies that could aid millions affected by these conditions. Her research exemplifies how foundational science can lead to critical advancements in medical treatments, underlining the necessity for continued support in this area.