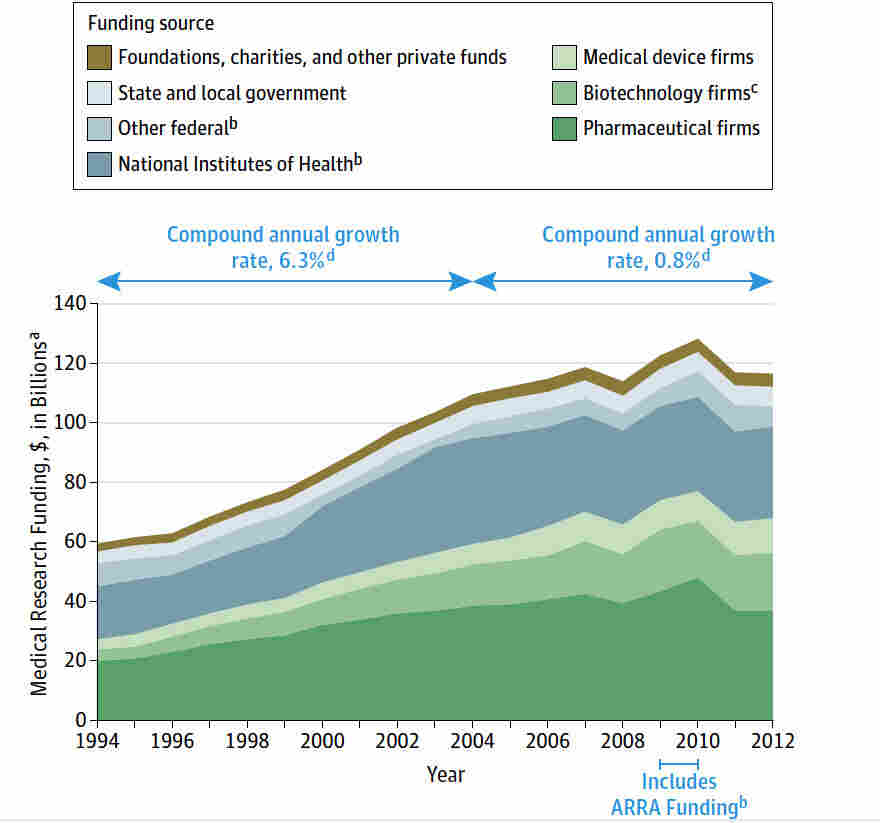
Medical research funding plays a crucial role in advancing healthcare and enhancing patient safety in research. As federal funding, particularly from the NIH, faces uncertainties, the impact on clinical trials and oversight mechanisms becomes significant. The integrity of research ethics is at stake without adequate financing, affecting the essential role of institutional review boards (IRBs) in safeguarding participants’ rights. These boards ensure that rigorous standards are upheld throughout studies, promoting transparency and accountability. The ramifications of funding cuts not only hinder ongoing research but also amplify public mistrust, ultimately jeopardizing the safety and well-being of individuals participating in medical studies.
Financial support for healthcare investigations is essential for innovation in medical science, especially as it relates to patient welfare and ethical oversight. The cessation of substantial grants jeopardizes critical roles such as those played by institutional review boards (IRBs), which are responsible for meticulously evaluating the safety and ethical implications of studies. When research funding dwindles, the ability to properly oversee clinical trials diminishes, potentially compromising participant safety and the integrity of research outcomes. Moreover, the lack of adequate resources can hinder collaboration across institutions, limiting advancements that ensure the well-being of patients involved in medical trials. As such, robust investment in this sector is indispensable for fostering trust and ensuring the protection of all individuals engaged in healthcare research.
Impact of NIH Funding on Patient Safety in Research
The National Institutes of Health (NIH) play a vital role in ensuring patient safety within medical research through their funding mechanisms. NIH funding directly supports rigorous protocols that protect human subjects and promote ethical research practices. This includes mandates for institutional review board (IRB) oversight, which ensures that research studies comply with ethical standards and regulations designed to safeguard participant rights. By providing financial resources, NIH facilitates the rigorous ethical review of research proposals, ensuring that potential risks are identified and mitigated before any study can commence.
Moreover, NIH-funded research often leads to the development of groundbreaking medical advancements that can dramatically impact patient care. The requirement for IRB review is not merely bureaucratic; it is a fundamental aspect of maintaining participant safety and trust in clinical trials. As researchers grapple with the complexities of modern medical inquiries, NIH funding enables them to uphold the highest standards of patient safety while fostering innovative, ethical research approaches that ultimately benefit society.
The Role of Institutional Review Boards (IRBs) in Protecting Research Participants
Institutional Review Boards (IRBs) serve a critical function within the landscape of medical research, particularly in ensuring the safety and well-being of study participants. By rigorously reviewing research proposals, IRBs assess various facets of studies, including recruitment strategies, informed consent processes, and potential risks associated with the research. This process is essential in upholding ethical standards and ensuring that participants are fully aware of their involvement and any potential risks involved. IRBs act as gatekeepers, ensuring that research that is conducted honors the dignity and safety of its participants.
Additionally, IRBs foster a culture of ethical research practices by offering training and guidance to researchers. This support helps researchers navigate complex ethical dilemmas that may arise during their studies. Without the essential oversight of IRBs, the potential for inadvertent harm to participants could increase, jeopardizing not only the integrity of individual studies but also the trust that the public places in medical research overall. The collaborative nature of the IRB process reinforces the commitment to ethical standards, ensuring that researchers are held accountable for their responsibilities towards participant safety.
Ethics in Medical Research: The Crucial Need for Oversight
Ethics form the backbone of medical research, particularly when it involves human subjects. A strong ethical framework is necessary to protect the rights and welfare of individuals who contribute to scientific progress through their participation. Historical abuses in research have underscored the need for stringent oversight to prevent violations of human rights and to maintain public trust in scientific inquiry. Regulatory bodies and ethical guidelines, such as those enforced by IRBs, are crucial in ensuring that research is conducted responsibly and that participants are treated with respect and integrity.
Research ethics not only safeguard participants but also enhance the quality and credibility of scientific studies. When researchers adhere to ethical guidelines, they are more likely to produce valid, reliable results that can be generalized to broader populations. Ethical considerations also promote transparency and accountability, which are essential for fostering public confidence in the research process. As the landscape of medical research evolves, maintaining a robust ethical framework will be vital in guiding researchers and protecting those who volunteer for studies.
Funding Cuts and Their Consequences on Research Oversight
Funding cuts to medical research have significant repercussions for research oversight and patient safety. When federal grants are frozen or eliminated, as seen with the recent $2 billion stop-work order affecting Harvard, the ability to conduct vital research is severely hampered. These financial constraints can disrupt ongoing research projects, delay the initiation of new studies, and limit access to necessary resources for those conducting IRB reviews. This cascading effect ultimately threatens the security and safety of research participants.
In addition to jeopardizing patient safety, funding cuts can erode public trust in the research community. Communities that participate in research studies may become skeptical of the intentions and capabilities of researchers, particularly when they observe that funding cuts lead to disruptions in studies designed to improve health outcomes. Maintaining robust funding for medical research is not just about exploring new scientific inquiries; it is also about ensuring that existing projects can be carried out with the appropriate oversight and support necessary to protect participants effectively.
The Importance of Collaborative Research in Medical Trials
Collaboration among research institutions is crucial for advancing medical knowledge and patient care. Initiatives like the SMART IRB demonstrate the power of collaborative efforts in enhancing research efficiency and oversight across multiple sites. By streamlining the IRB process, researchers can work together more effectively, reducing redundancies and accelerating the move from research to clinical application. This cooperation is vital for complex health issues that require input from diverse fields, ultimately benefiting the patients who rely on these advancements.
However, funding cuts compromise the ability of research institutions to collaborate effectively. When institutions cannot access the necessary resources to support collaborative initiatives, the speed and scope of scientific discovery can be significantly hindered. This impacts not only the research community but also the patients who depend on groundbreaking treatments that stem from collaborative efforts. Investing in research funding is essential to maintaining a robust network of cooperation among institutions dedicated to improving health care outcomes.
Understanding Informed Consent in Clinical Research
Informed consent is a cornerstone of ethical medical research. It ensures that participants fully understand the implications of their involvement in a study, including potential risks, benefits, and the nature of the research itself. IRBs play an essential role in reviewing and approving informed consent documents to ensure they are clear, comprehensive, and comprehensible to participants. Without rigorous processes in place to secure truly informed consent, the ethical integrity of the research can be called into question.
The emphasis on informed consent also reflects a broader commitment to participant autonomy and empowerment. Involving individuals in decisions about their participation not only protects their rights but also fosters trust in the research process. Those who feel informed and respected are more likely to engage in research, positively impacting the ability to recruit diverse and representative participant pools. This trust is especially crucial in studies that address sensitive health issues, where participant engagement is vital for generating meaningful and applicable results.
Challenges in Clinical Trial Oversight
Clinical trial oversight faces numerous challenges, particularly in light of funding cuts and the evolving landscape of medical research. As noted with the stop-work order impacting various studies, the complexity of managing multiple sites and ensuring rigorous oversight can be overwhelming without adequate resources. Trials involving hundreds of participants and numerous institutions necessitate comprehensive oversight to monitor participant safety and compliance with ethical standards. Disruptions in funding can hinder these essential oversight functions.
Moreover, the rapid advancement of technology and research methodologies presents additional hurdles that require constant updates to oversight practices. IRBs and related oversight committees must remain vigilant and adaptable to ensure that new research techniques uphold participant safety and ethical standards. As challenges continue to mount, ensuring adequate funding for oversight is crucial for maintaining the integrity of clinical trials and safeguarding the rights of all individuals involved.
The Future of Medical Research Funding
The future of medical research funding remains precarious, particularly in light of recent belt-tightening measures. It is vital for policymakers to recognize the importance of sustained investment in research initiatives that protect patient safety and uphold ethical standards. Funding must not only aim at scientific advancement but also ensure the robustness of systems that govern research practices, particularly those involving human subjects. Continuous support for NIH and similar funding mechanisms is essential to nurturing an environment conducive to ethical research.
Furthermore, a shift towards prioritizing collaborative funding programs could enhance the quality and efficiency of research. By pooling resources and expertise, institutions can work together to tackle the world’s most pressing health challenges, ultimately benefiting patient safety and health outcomes. Advocating for increased funding streams and collaborative initiatives is crucial as we strive for a future where medical research not only thrives but also emphasizes the importance of ethical oversight and participant protection.
Frequently Asked Questions
What is the impact of NIH funding on patient safety in medical research?
NIH funding plays a vital role in ensuring patient safety in medical research by requiring that studies involving human participants undergo rigorous review and oversight by institutional review boards (IRBs). These IRBs help protect the rights and welfare of participants, ensuring compliance with federal regulations and ethical standards. The financial support from NIH enables research institutions to maintain robust IRB operations, which are essential for safeguarding patients involved in clinical studies.
How does the role of IRBs contribute to the ethics of medical research funding?
IRBs are instrumental in upholding research ethics when it comes to medical research funding. They review research proposals meticulously to ensure participant safety, assess potential risks, and ensure informed consent is obtained properly. The existence of IRBs, supported by funding, enforces a system of checks and balances, ensuring that ethical standards are maintained throughout the research process and that patients are not put at unnecessary risk.
In what ways can funding cuts affect clinical trial oversight and research ethics?
Funding cuts can severely undermine clinical trial oversight and research ethics by limiting the resources available for IRBs and leading to delays or cancellations of essential studies. When financial support is frozen or withdrawn, it can hinder the ability of research institutions to conduct thorough reviews and monitoring of ongoing research, ultimately jeopardizing the safety and trust of patients participating in clinical trials.
What are the broader implications of medical research funding on patient safety?
The implications of medical research funding on patient safety are significant. Adequate funding not only supports the operational strength of IRBs but also fosters a culture of compliance with ethical standards, enabling researchers to prioritize patient rights and welfare. Conversely, funding disruptions can diminish the oversight capabilities needed to protect patients, potentially leading to increased risk and harm from unmonitored or poorly regulated studies.
How does the SMART IRB system improve the efficiency of medical research funding and patient safety?
The SMART IRB system streamlines the review process for multisite clinical studies, allowing institutions to collaborate more efficiently. This collaboration is crucial for advancing medical research while ensuring patient safety. By reducing barriers to initiating studies and facilitating shared oversight, the SMART IRB framework enhances the overall effectiveness of research funding and ensures a consistent ethical approach to protecting research participants.
Why is the transparency of medical research funding essential for enhancing public trust in patient safety?
Transparency in medical research funding is essential for fostering public trust in patient safety. When funding sources and the allocation of resources are clear, it reinforces the integrity of the research process. Public trust is enhanced when patients know that studies are conducted ethically, with sufficient oversight and funding backing the necessary safety protocols.
| Key Point | Details |
|---|---|
| Federal Funding Freeze | The Trump administration froze over $2 billion in federal research grants to Harvard, affecting patient safety in medical research. |
| SMART IRB Overview | SMART IRB is a national system that enhances oversight of medical research across multiple sites, managed by Harvard Catalyst. |
| Role of IRBs | IRBs review research proposals to protect participants, ensuring compliance with laws and ethical standards. |
| Impact of Historical Issues | Historical unethical medical practices highlight the need for stringent oversight in research to protect participant safety. |
| Consequences of Funding Cuts | Funding cuts jeopardize research participants and undermine trust in the research community, delaying critical studies. |
| Role of Harvard Medical School | Despite funding cuts, Harvard Medical School continues to support collaborative research efforts vital for patient safety. |
Summary
Medical research funding is crucial for the safety and effective oversight of clinical trials involving human participants. The recent halt in funding, particularly impacting institutions like Harvard, threatens patient rights and welfare, jeopardizing ongoing studies and fostering public skepticism. Such a disruption can adversely affect the careful checks and balances established through institutional review boards (IRBs) and collaborative research efforts like SMART IRB, which are essential in ensuring ethical compliance and participant protection. As we navigate these challenges, it is imperative to recognize the critical role that continuous and adequate funding plays in safeguarding the integrity of medical research and the health of the population.