
CRISPR Gene Editing: Ethical Dilemmas and Sickle Cell Treatment
CRISPR gene editing represents a revolutionary advancement in biotechnology, providing unprecedented ability to modify genetic material with precision and ease. The implications of CRISPR technology are vast, especially in the context of treating genetic disorders such as sickle cell disease. However, as we stand on the cusp of these groundbreaking possibilities, critical discussions around gene editing ethics emerge, challenging us to consider the ramifications of human intervention in our genetic framework. The promise of eradicating hereditary diseases raises vital questions about health equity issues and the risks inherent to altering the human genome. As the exploration of bioethics in medicine evolves, society must grapple with whether we should wield such powerful tools and to what extent, ensuring that we do so in a manner that respects human dignity and equality.
The advent of gene editing technologies, particularly those leveraging CRISPR methodologies, has sparked significant debate in scientific and ethical communities. These innovations allow for targeted modification of DNA, leading to potential breakthroughs in medical treatments for conditions like hereditary blood disorders. Nonetheless, the conversation surrounding this powerful tool encompasses an array of ethical dilemmas that must be navigated carefully. Topics such as the responsibilities of altering human genetics and the implications for health equity are increasingly pertinent as this field develops. As we delve into discussions on bioethical standards, it is imperative to consider both the potential benefits and the risks associated with gene modification.
Understanding CRISPR Gene Editing
CRISPR gene editing refers to a revolutionary technology that can modify DNA within living organisms, enabling the precise alteration of genetic materials. This innovative approach allows researchers to insert, delete, or modify specific genes, opening doors to potential cures for genetic disorders, including sickle cell disease. The capability of CRISPR to edit somatic and germline cells presents a remarkable opportunity for medical science, enabling targeted therapies that were once unimaginable.
However, the implementation of CRISPR gene editing is not without controversy. It raises significant ethical questions related to the nature of human intervention in genetic makeup and the long-term implications of such modifications. As we grapple with these advancements, it is important to establish a framework that addresses the ethical considerations surrounding gene editing and its potential to alter the human condition. Discussions in bioethics and medicine continue to evolve as new cases of CRISPR applications emerge.
Ethical Considerations in Gene Editing
The ethical implications of gene editing become stark when considering potential applications beyond treating diseases. For instance, while editing genes to eliminate sickle cell disease is seen as a noble pursuit, it prompts a deeper inquiry into what other traits could be considered for modification. Ethical discourse around gene editing emphasizes the importance of distinguishing between curing diseases and enhancing human traits, raising questions about societal values and norms regarding disability and human diversity.
As experts like Neal Baer stress, decisions around which conditions warrant gene editing require careful consideration. Will the capability to edit genes for non-life-threatening conditions, such as certain syndromes, create a slippery slope towards eugenics? The medical community and society at large must navigate these dire ethical waters to ensure that gene editing serves the common good without infringing on individual rights and societal equity.
CRISPR Technology and Health Equity Issues
One of the pressing concerns surrounding CRISPR technology is the issue of health equity. The current costs associated with gene therapies, such as the sickle cell ‘cure’ priced at roughly $2.2 million, raise alarms about access and availability for marginalized communities. In a world where medical advancements are often economically stratified, the risk of widening the health gap is prevalent. Will those from lower socioeconomic backgrounds continue to have limited access to transformative treatments?
Furthermore, discussions led by experts like Rebecca Weintraub Brendel highlight the necessity of incorporating health justice perspectives into the innovation process. The benefits of CRISPR technology must not only be accessible to those who can afford them but should ideally reach all populations impacted by genetic diseases, ensuring that the promise of gene editing contributes to reducing, rather than exacerbating, health disparities.
The Complex Reality of Genetic Modification
The reality of genetic modification is layered and complicated. Tools like CRISPR give scientists the ability to make profound changes to DNA, but with great power comes great responsibility. There are numerous examples of unintended consequences when altering genes, as elucidated by Baer. Altering genes linked to diseases may affect other biological functions, raising questions about the unforeseen impacts such changes might have on human health over generations.
Examining the complexities of genetic modification also leads us to consider the balance between innovation and regulation. Who will oversee the application of CRISPR technology, especially in countries with lax regulations? Without international consensus on bioethics in medicine, the risk of unethical applications, including designer babies or enhancements for non-medical reasons, looms large.
Bioethics in Gene Editing Research
Bioethics plays a pivotal role in guiding discussions and policies surrounding gene editing research. The field intersects with CRISPR technology, prompting essential conversations about consent, the definition of disease, and the societal implications of genetic alterations. Ethical considerations not only involve the immediate effects on individuals subjected to such treatments but also the long-term ramifications for humanity as a whole.
Research in bioethics also seeks to answer challenging questions regarding ‘who decides’ the acceptable boundaries for gene editing. Should decisions rest solely in the hands of scientists and medical professionals, or should voices from ethicists, patients, and society at large be equally considered? Ensuring diverse perspectives in bioethics discussions may contribute to more equitable and just applications of gene editing technologies, promoting public trust and acceptance.
Impact of Gene Editing on Future Medical Treatments
The introduction of gene editing technologies such as CRISPR holds the promise of transformative changes in how we understand and treat medical conditions. Already, experiments with CRISPR have shown potential in curing genetic diseases, suggesting that many conditions previously deemed untreatable might soon be addressed on a genomic level. The future of medicine could be reshaped significantly as we harness the power of gene editing.
However, as we embrace the potential benefits, we must remain vigilant regarding potential abuses of this technology. The possibility of using CRISPR to create genetic advantages raises ethical dilemmas about fairness, especially in competitive environments such as academics and sports. The challenge will be to realize the benefits of gene editing while ensuring equitable access and preventing the deepening of existing societal inequalities.
Concerns Over Designer Babies and Parental Choice
As CRISPR technology progresses, one of the most contentious issues is the idea of ‘designer babies.’ This concept involves using gene editing to select or enhance specific traits in offspring, which raises significant ethical and moral dilemmas. The implications of parents actively choosing traits such as intelligence, appearance, or health status can fundamentally alter societal perceptions of disability and diversity, leading to a homogenized view of human potential.
Baer’s inquiries about parental rights to make genetic decisions underline the ethical complexities surrounding reproductive technologies. While some might argue for parental freedom to choose traits for their children, others warn that such choices can reinforce stereotypes and stigmas related to specific disabilities. Balancing parental autonomy and ethical responsibility will be critical as society navigates this rapidly evolving landscape.
The Role of Legislation in Gene Editing
As gene editing technologies such as CRISPR make significant advancements, legislative frameworks must evolve to address the associated ethical, safety, and health equity concerns. Current laws must adapt to encompass the complexities of editing genetic material, especially when it comes to human subjects. The development of comprehensive legislation that governs gene editing practices can help mitigate potential risks and abuses, ensuring that scientific progress does not outpace ethical considerations.
Legislation should focus not only on the safety of gene editing but also on promoting access to therapies derived from these innovations. Keeping in mind the health equity issues raised during discussions about CRISPR, effective legislation can ensure that the benefits of gene editing are distributed fairly across all societal sectors, providing safeguards against the commodification of human life.
Public Perception and Acceptance of CRISPR Technology
The public’s perception of CRISPR technology plays a crucial role in how it is adopted and implemented in society. As with any groundbreaking innovation, public understanding and acceptance can vary widely based on cultural, ethical, and personal beliefs. Media portrayals, educational efforts, and transparent conversations about the benefits and risks associated with gene editing are vital in shaping informed public opinion.
Incorporating diverse societal viewpoints in conversations about CRISPR can foster engagement and trust. As public awareness about gene editing expands, harnessing dialogue around ethical implications and personal experiences related to genetic diseases can shift perceptions toward a more nuanced understanding. Open discussions can bridge gaps between scientific communities and the public, ensuring that advancements in gene editing are met with thoughtful consideration.
Frequently Asked Questions
What are the ethical implications of CRISPR gene editing?
CRISPR gene editing raises significant ethical questions, particularly surrounding the modification of human characteristics and diseases. Discussions include the right to edit genes associated with conditions like Down syndrome, and who should make these decisions. Issues of consent, especially for future generations, and the potential for misuse of the technology for non-therapeutic modifications are central in the conversation about gene editing ethics.
How does CRISPR technology contribute to the treatment of sickle cell disease?
CRISPR technology provides a promising avenue for treating sickle cell disease by enabling targeted editing of genes responsible for the disorder. By modifying somatic cells, researchers can effectively remove the genetic mutations that cause sickle cell, leading to potential cures. However, the high cost associated with these treatments raises questions about accessibility and health equity issues that must be addressed.
What role does bioethics in medicine play in the advancements of CRISPR gene editing?
Bioethics in medicine is crucial in guiding the responsible use of CRISPR gene editing technologies. As scientific advancements progress, bioethicists evaluate the implications of gene editing on patient consent, societal norms, and potential health disparities. These ethical considerations aim to ensure that innovations in CRISPR technology do not exacerbate inequities in healthcare access.
What are the health equity issues related to CRISPR gene editing treatments?
Health equity issues around CRISPR gene editing treatments focus on the accessibility and affordability of genetic therapies. With treatments like sickle cell disease cures costing millions, there is a concern about who will benefit from these advancements. Ongoing discussions emphasize the need for equitable distribution of CRISPR-based therapies to prevent further widening of health disparities among different populations.
How might CRISPR technology affect parental decision-making regarding children’s traits?
CRISPR technology raises complex questions about parental decision-making in selecting traits for their children. As gene editing becomes more accessible, parents may feel empowered, or pressured, to modify genes to avoid potential disabilities or enhance certain attributes. This leads to ethical debates on the implications of such decisions, including the potential loss of genetic diversity and the societal expectations placed on future generations.
| Key Topic | Details |
|---|---|
| Introduction to CRISPR | Discussion on the potential and ethical issues raised by CRISPR technology. |
| Neal Baer’s Experience | Shared personal experience treating sick children and how CRISPR offers hope for curing diseases like sickle cell anemia. |
| Ethical Dilemmas | Questions raised include the appropriateness of editing genes for conditions that do not threaten life, and issues of choice and parental rights. |
| Costs and Access | The high cost of treatments like CRISPR for sickle cell disease ($2.2 million) raises concerns regarding health equity and access. |
| Oversight Issues | Concerns about the lack of regulation in countries like Russia and China regarding genetic modifications. |
| Unintended Consequences | Gene editing may have complex unforeseen effects due to the interconnected nature of genes. |
Summary
CRISPR gene editing represents a groundbreaking advancement in biotechnology, promising potential cures for diseases while also raising critical ethical questions. As highlighted in discussions surrounding this technology, the ability to modify genes for conditions like sickle cell anemia brings both excitement and apprehension. The implications of gene editing stretch beyond mere healthcare advantages, touching on moral dilemmas about parental decisions, equity in access to expensive treatments, and the unpredictable outcomes of altering our genetic code. Thus, while CRISPR holds the promise of revolutionary change in medicine, it is essential to navigate these ethical waters thoughtfully.
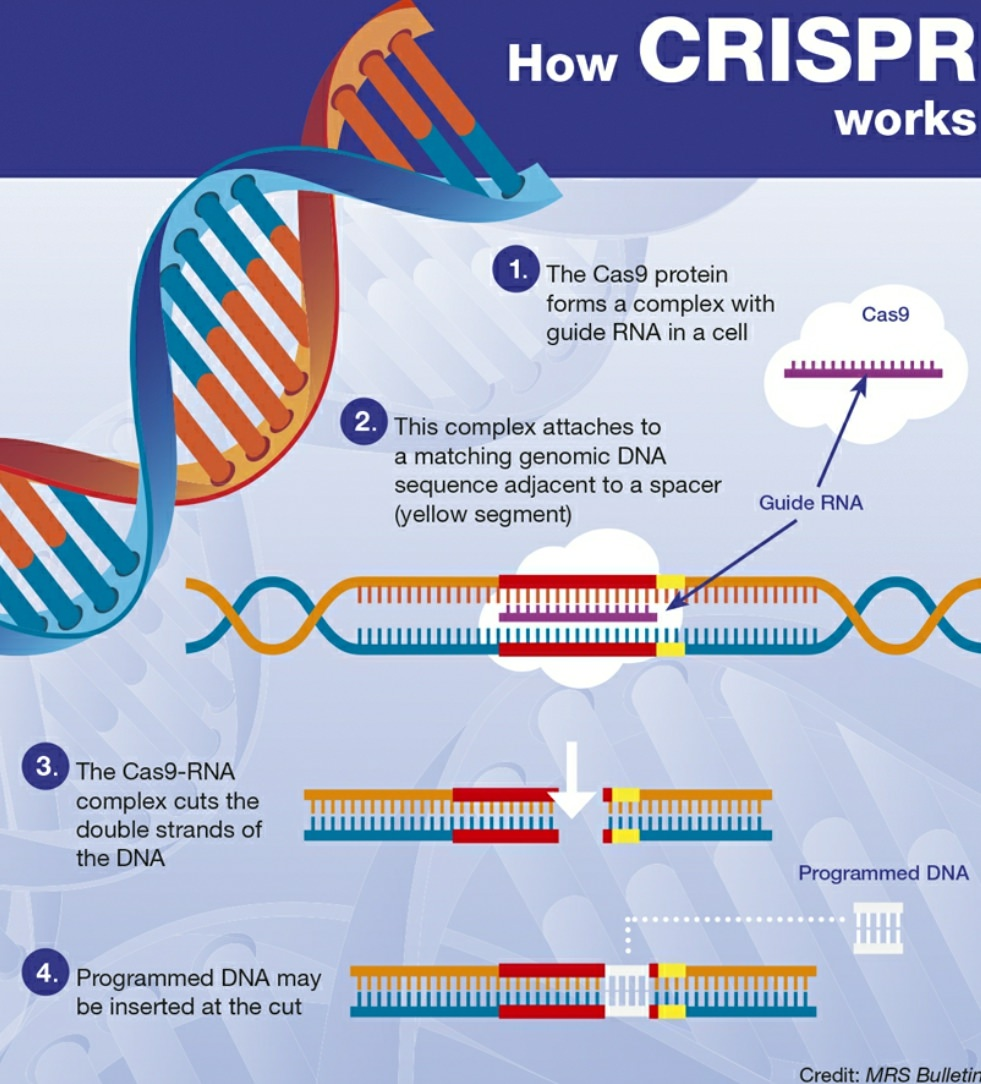
CRISPR Technology: The Ethics Behind Gene Editing
CRISPR technology is revolutionizing the field of gene editing, offering unprecedented capabilities to manipulate genetic material in pursuit of curing diseases. This powerful tool can precisely alter genes to eliminate ailments such as sickle cell anemia, raising important questions about the implications of such changes. As researchers examine the potential for CRISPR to reshape human health, they must also grapple with the ethics of gene editing and the broader societal impact on health equity. The conversation surrounding the latest in biomedical ethics emphasizes not just the science, but also the responsibility that comes with such potent capabilities. Amidst the promise of advanced treatments, the ongoing debate reflects the tension between innovation and the moral considerations that must guide these scientific breakthroughs.
Gene editing, particularly through advanced techniques like CRISPR, represents a frontier in modern medicine, holding the key to potentially eradicating genetic disorders. This groundbreaking technology allows scientists to tailor genetic sequences with precision, sparking discussions about its ethical dimensions and the ramifications for health equity in diverse communities. The term ‘genetic modification’ encapsulates the ongoing discourse about our ability and responsibility to alter human genetics, especially in cases like the treatment of sickle cell disease. As stakeholders in the medical field ponder the implications of these advancements, they must navigate complex issues related to biomedical ethics and the potential societal divide created by access to such innovative therapies. In exploring these themes, it becomes clear that the future of gene editing is as much about moral choices as it is about scientific progress.
Understanding CRISPR Technology in Gene Editing
CRISPR technology represents a revolutionary advancement in the field of gene editing, providing scientists with tools to modify the DNA of living organisms with unprecedented precision. This innovative system was initially derived from a bacterial immune response, allowing researchers to target specific sequences within the genome. By utilizing CRISPR’s RNA-guided mechanism, scholars can now cut out faulty genes and insert correct versions, opening the door to potential cures for genetic disorders such as sickle cell anemia. Moreover, the implications of such technology extend beyond mere correction; they raise significant questions about the essence of genetic modification and its ethical ramifications.
As CRISPR technology continues to evolve, it emphasizes the need to address both the scientific and ethical concerns that accompany gene editing. The capability to eliminate devastating genetic diseases is undoubtedly appealing; however, it prompts a broader dialogue about the boundaries of genetic intervention. For instance, what are the responsibilities of researchers and clinicians in deciding which traits to alter? With possibilities for germline editing, decisions made today could affect generations to come, raising concerns over unintended consequences and ethical considerations surrounding health equity and access.
Ethics of Gene Editing and Health Equity
The ethics of gene editing are at the forefront of discussions regarding CRISPR technology and its applications. As highlighted in Neal Baer’s talk, one key ethical concern is the potential for socioeconomic disparities to widen if such technologies become available only to those who can afford them. For instance, the sickle cell treatment currently costs around $2.2 million, creating questions about who truly benefits from these advancements. The disparity in access to cutting-edge biomedical technology not only poses challenges for affected populations but also raises fundamental issues of justice and health equity in medical practice.
Moreover, as gene editing techniques continue to become more refined, the ethical landscape expands to encompass diverse issues, from the potential for designer babies to the implications of editing health-related traits versus non-pathological human variations. The discussion encourages a holistic approach to biomedical ethics, urging policymakers and the scientific community to consider the broader societal impacts of gene editing. Ensuring that advancements in CRISPR technology contribute equitably to public health will require transparency, comprehensive oversight, and a commitment to addressing the needs of marginalized communities.
The Promise of Curing Sickle Cell Disease
One of the most significant promises of CRISPR technology lies in its potential to provide cures for genetic conditions such as sickle cell disease. This painful disorder affects approximately 100,000 individuals in the United States alone, leading to severe health complications. By employing CRISPR to alter somatic cells, researchers have been able to reverse the genetic mutations that cause sickle cell disease, offering hope to many families who have endured the consequences of this condition. The prospect of a functional cure brings forth an optimistic outlook for patients and medical professionals alike.
Despite the potential benefits of curing sickle cell disease, ethical questions arise about the implications and accessibility of such treatments. The high cost associated with gene modification therapies leads to disparities within healthcare, creating challenges regarding who has the right to receive potentially life-saving procedures. Moreover, the ethical dilemma intensifies when considering the extent to which parents or guardians should exert influence over genetic traits for their children. While the promise of CRISPR technology to eradicate diseases is compelling, it is essential to engage in continuous dialogue surrounding equity and ethics in healthcare solutions.
The Role of Biomedical Ethics in Gene Editing
Biomedical ethics plays a crucial role in navigating the complexities of gene editing, especially as technologies like CRISPR become more prevalent in the medical field. Discussions surrounding gene editing often invoke questions related to consent, the role of parents and guardians, and the implications of altering genetic traits. With advancements allowing for modifications on both somatic and germline levels, there is a pressing need for a robust ethical framework to guide research and clinical applications. Ensuring that patients and families are adequately informed about the ramifications of gene editing is paramount.
Furthermore, the principles of biomedical ethics—beneficence, non-maleficence, autonomy, and justice—serve as a compass to navigate the challenges associated with gene editing. As researchers and clinicians grapple with the application of CRISPR technology, they must consider not only the medical outcomes but also the broader social impacts of their work. This commitment to ethical responsibility will be essential in fostering public trust, promoting health equity, and ensuring that the benefits of gene editing technologies are distributed fairly among all populations.
Risks and Unintended Consequences of Gene Editing
While CRISPR technology has opened new avenues for genetic interventions, it is essential to recognize the potential risks and unintended consequences that may accompany gene editing. As illustrated by Neal Baer’s discussion, the modification of genes can lead to unforeseen outcomes due to the complex interactions of genetic components within the body. For example, editing a single gene intended to lower LDL cholesterol may inadvertently affect other biological processes, highlighting the intricate nature of human genetics and the need for comprehensive research before widespread applications are made.
In addition to biological risks, gene editing generates concerns regarding ethical governance. The absence of universal regulatory standards grants rooms for potential abuses, especially in countries with less stringent oversight. Speculative scenarios, such as genetic enhancements for military purposes or designer babies, provoke important ethical questions surrounding the implications of manipulating human genetics. As society navigates the exhilarating possibilities of CRISPR technology, it is crucial to maintain a vigilant approach toward understanding both its potential benefits and risks.
The Debate Over Genetic Modification of Traits
The possibility of using CRISPR technology to modify traits presents a moral quandary that extends beyond mere medical necessity. As Baer mentions, ethical dilemmas arise when considering whether it is appropriate to edit genes associated with conditions that are not life-threatening, such as Down syndrome. This raises poignant questions: who gets to determine which traits are desirable or undesirable? Allowing parents or society at large to dictate genetic attributes could lead to increased social pressure and stigmatization of those who do not conform to certain standards. Such considerations warrant thorough ethical deliberation.
Moreover, the notion of modifying non-pathological traits could pave the way for a culture that values certain characteristics over others, heightening societal divisions. As gene editing technology progresses, discussions must shift to include not only the technical abilities of such advancements but also the moral implications and potential societal repercussions. Encouraging responsible discourse around gene editing is essential for shaping a future that values diversity and human variation rather than one that seeks uniformity through genetic alteration.
Oversight and Regulation of Gene Editing
The question of oversight in gene editing practices, particularly with technologies like CRISPR, is increasingly pertinent as the field rapidly evolves. Baer’s remarks about the challenges in monitoring international research practices shine a light on a critical oversight gap. For example, while certain countries have robust frameworks to regulate gene editing, others may lack stringent guidelines, allowing for risky experimentation without proper checks. The international community must collaborate to establish comprehensive regulatory frameworks that ensure the safety and ethical application of gene editing technologies across borders.
Moreover, effective oversight is not limited to monitoring research and clinical applications but also extends to public education and engagement. Informing the public about the risks and benefits of gene editing is crucial for fostering informed consent and promoting responsible use of technology. By creating open channels of communication between scientists, ethicists, and the public, we can work toward establishing a shared understanding of gene editing’s potential and pitfalls. This collaborative approach will help ensure that advancements in CRISPR technology are realized responsibly and ethically, benefiting all segments of society.
Cultural Perspectives on Genetic Variation
Cultural perspectives on genetic variation play a significant role in shaping societal attitudes toward gene editing technologies like CRISPR. As indicated in Baer’s discussion, the perceptions surrounding conditions like deafness or albinism as aspects of human diversity rather than pathology challenge the notion of ‘fixing’ genetic traits. Advocates from various cultural backgrounds emphasize the importance of recognizing the value of differences, which should lead to a broader understanding of genetic diversity as an enriching facet of the human experience rather than a defect needing correction.
These cultural perspectives underscore the necessity of inclusive dialogue when addressing the implications of gene editing. As society grapples with the advancements brought forth by CRISPR technology, it is vital to incorporate diverse voices and champion the need for respect toward all forms of human variation. Through this lens, gene editing can be approached not just as a medical tool for enhancement, but as a means to engage in deeper conversations about identity, belonging, and the ethical limits of human intervention in the natural world.
Future Directions in Gene Editing Research
The future of gene editing research will hinge on both technological advancements and the ethical frameworks developed to guide them. As scientists continue to refine CRISPR technology, exploring its applications across various fields—from agriculture to medicine—the potential for groundbreaking discoveries is vast. However, the directions taken in this research must include a thorough consideration of the associated ethical implications, especially with regard to health equity and access to these scientific innovations.
Additionally, interdisciplinary collaboration among researchers, ethicists, and community stakeholders will be essential for paving a responsible path forward in gene editing. Engaging diverse perspectives ensures that advancements in CRISPR technology serve the greater good and do not exacerbate existing social inequalities. As we look toward the future of gene editing, the ultimate goal should be to harness this powerful technology in a manner that is ethical, equitable, and beneficial to all members of society.
Frequently Asked Questions
What is CRISPR technology and how does it relate to gene editing?
CRISPR technology is a revolutionary gene editing tool that allows scientists to precisely alter DNA sequences within living organisms. It utilizes a natural defense mechanism found in bacteria, enabling targeted modification of genes, which has vast implications for medical research, including the potential treatment of genetic disorders and diseases.
What are the ethical concerns surrounding CRISPR technology in gene editing?
The ethics of gene editing with CRISPR technology raise significant questions about the implications of altering human DNA. Ethical concerns include the right to make decisions about genetic modifications, potential discriminatory practices against certain genetic traits, and the responsibilities of scientists in ensuring fair access to gene editing treatments.
How can CRISPR technology potentially cure sickle cell disease?
CRISPR technology has the capability to edit somatic cells to correct the genetic mutations causing sickle cell disease. By precisely targeting and modifying the defective genes in patients, scientists can provide a potential cure, allowing individuals to recover from the painful symptoms associated with this genetic disorder.
What is the relationship between health equity and CRISPR gene editing?
Health equity is a critical consideration in the context of CRISPR gene editing, as access to these innovative treatments may exacerbate existing healthcare disparities. The high costs associated with gene therapies can limit availability for marginalized populations, raising ethical concerns about who benefits from advancements in biomedical technologies.
What are the potential unintended consequences of CRISPR gene editing?
While CRISPR gene editing presents formidable possibilities, it can also lead to unintended consequences due to the complex interactions within the genome. Altering one gene may inadvertently affect other genes, leading to unforeseen health issues or mutations that could have lasting effects, highlighting the need for careful oversight and ethical consideration in its applications.
Who should make decisions about gene editing in humans using CRISPR technology?
Decisions about CRISPR-based gene editing in humans should involve a multidisciplinary approach that includes ethicists, scientists, policymakers, and public input. The complexity of gene modifications necessitates a careful balance of scientific knowledge and public ethical standards to determine what is acceptable and beneficial for society.
What role does oversight play in the use of CRISPR gene editing technologies?
Oversight is crucial in the use of CRISPR gene editing technologies to ensure ethical practices and prevent misuse. Regulatory bodies must establish guidelines for research, clinical applications, and modifications to human germline cells, ensuring that the benefits of gene editing are achieved responsibly while minimizing harm and ethical breaches.
| Key Point | Details |
|---|---|
| Ethical Dilemma | The central question posed is about the ethical implications of changing human differences through CRISPR. |
| Potential Cures | CRISPR may offer cures for diseases like sickle cell anemia, raising questions about the morality of such interventions. |
| Costs and Access | Curing sickle cell can cost around $2.2 million, raising issues of health equity and access. |
| Social Implications | What if parents choose genetic modifications based on personal preference? This opens discussions about parental rights versus child autonomy. |
| Oversight and Regulation | Concerns exist regarding who regulates gene editing and potential for misuse in countries with less oversight. |
| Unintended Consequences | Gene editing could lead to unforeseen health issues due to the complex nature of genetic interactions. |
Summary
CRISPR technology is revolutionizing the field of medicine by offering potential cures for genetic diseases, but it also raises significant ethical questions. As scientists explore the possibilities offered by CRISPR, it is essential to navigate the moral landscape carefully, ensuring that such advancements do not exacerbate inequalities or lead to unintended consequences. Balancing the benefits of curing illnesses like sickle cell disease with the ethical implications of genetic modifications is critical for the responsible use of this powerful technology.
Gene Editing Advancements: A Breakthrough in Medicine
Gene editing advancements are at the forefront of revolutionary changes in medicine, offering unprecedented opportunities to treat and potentially cure genetic diseases. Recent developments in CRISPR technology, along with innovations such as base editing and prime editing, have significantly enhanced our ability to alter genetic material with precision. Pioneered by experts like David Liu, these techniques allow scientists to directly address the mutations responsible for various ailments, marking a new era in therapeutic interventions. As clinical trials continue to demonstrate remarkable outcomes, it is clear that these advancements are transforming how we understand and treat hereditary conditions. The journey of gene editing is not just a scientific endeavor; it embodies hope for millions affected by genetic disorders worldwide.
The field of genetic modification has witnessed groundbreaking progress that is reshaping the landscape of healthcare. Alternative methods, including genetic engineering and molecular editing technologies, are being developed to tackle the complexities of genetic disorders. Innovators in the scientific community, like those working with CRISPR-based systems, are leveraging their findings to create robust solutions tailored to individual genetic profiles. This shift towards precise genetic alterations signifies a major step in modern medicine, enhancing our ability to manage and potentially reverse the impact of genetic anomalies. As researchers unveil new possibilities, the implications for disease management and health outcomes are becoming increasingly promising.
The Rise of Gene Editing Advancements
In recent years, gene editing advancements have made remarkable strides, particularly with the introduction of base editing and prime editing technologies. These innovations have transformed how scientists approach genetic diseases that were once deemed untreatable. Base editing, which enables precise modifications at specific spots in DNA, allows for an unprecedented level of control over genetic alterations. This approach, developed by David Liu and his team, is demonstrating the capacity to correct mutations without the risks associated with traditional gene-editing methods, such as the CRISPR-Cas9 technique that cuts DNA strands.
Prime editing represents another significant leap in the field, functioning more like a word processor for DNA. It allows researchers to replace faulty DNA sequences with correct ones without causing double-strand breaks, which often lead to unintended consequences. This groundbreaking technology has opened new avenues for the treatment of a diverse range of genetic disorders. With ongoing clinical trials, we are witnessing the tangible impact of these gene editing advancements in therapeutic settings. Liu’s innovative work signifies not just a technological triumph but a beacon of hope for millions affected by genetic diseases.
The Impact of CRISPR Technology
CRISPR technology revolutionized genetic research when it was discovered that bacteria use it as a defense mechanism against viruses. This system provides a way to edit genes with incredible precision, and it sparked a wave of enthusiasm and exploration in the scientific community. David Liu’s contributions have expanded this foundation, moving beyond CRISPR’s original applications to refine methods of gene correction. The development of base editing and prime editing can be traced back to the insights gained from studying CRISPR, showcasing how basic scientific research can lead to groundbreaking technological advancements.
The implications of CRISPR are vast, impacting not only medicine but agricultural practices and biodiversity conservation as well. In the realm of genetic diseases, CRISPR-based technologies have the potential to address some of the most persistent issues in healthcare. Liu emphasizes that it’s vital for researchers to ensure the safety of these tools in clinical applications, as society grapples with ethical considerations and the potential for misuse. By navigating these challenges, the scientific community can harness the powers of CRISPR technology responsibly, leading to a healthier future for all.
Exploring Base Editing Techniques
Base editing is a transformative gene-editing technique that allows for targeted alteration of DNA bases without cutting the DNA strands. This approach enables scientists to rectify point mutations—variations in a single DNA base pair—that are responsible for many genetic diseases. By offering a more refined method of gene modulation, base editing minimizes errors and unintended consequences, thereby enhancing the safety of therapeutic interventions. As highlighted by David Liu’s work, transforming our understanding of how to manipulate DNA brings new hope to patients suffering from conditions like sickle cell disease and beta-thalassemia.
The precision of base editing makes it a compelling tool in the fight against genetic disorders. For example, recent clinical trials have demonstrated the effectiveness of this technique in ensuring long-term remission in patients previously reliant on medication. By correcting the root cause of these diseases at their genetic source, base editing holds the promise of transitioning from symptom management to disease resolution. As ongoing research continues to push the boundaries of what is possible, the future of base editing shines brightly in the landscape of genetic medicine.
Prime Editing: The Future of Genetic Correction
Prime editing is often touted as the next frontier in gene editing, thanks to its remarkable ability to introduce precise changes to DNA sequences. Liu’s pioneering work resulted in a technology that not only edits genes but also does so with unparalleled finesse. Unlike CRISPR and traditional gene-editing methods, prime editing acts like a word processor, allowing scientists to search for specific sequences of DNA and replace them accurately, addressing issues like extra or missing letters in the genetic code. This next-generation tool heralds a new era of possibilities for correcting inherited genetic disorders.
The implications of prime editing extend far beyond its technical capabilities. With ongoing research and clinical trials confirming its efficacy, prime editing is positioning itself as a cornerstone of future therapeutics. Patients with various genetic diseases may soon experience the benefits of these innovations, potentially leading to results that could redefine long-term outcomes. By marrying scientific rigor with technological advancement, prime editing is set to make substantial contributions to the field of genetic medicine, carrying the promise of transformative treatments to those in need.
Advances in Therapeutic Applications
The advances in gene editing techniques like base editing and prime editing are opening new doors for therapeutic applications. For instance, in clinical trials that utilize these technologies, patients are being treated for diseases previously deemed unmanageable. Notably, the case of Alyssa Tapley showcases how these innovations have resulted in successful remission of T-cell leukemia, providing a lifeline to many who suffer from genetic afflictions. By demonstrating tangible results, these therapeutic applications not only inspire confidence but also emphasize the importance of advancing gene editing methodologies.
There are at least 18 clinical trials currently underway that employ base editing or prime editing to treat various diseases. These trials are critical as they bridge the gap between laboratory research and real-world medical applications. Moving forward, the data gleaned from these studies will be essential in understanding the long-term impacts of gene editing on health outcomes. The advances made today are shaping the future of medicine, and the hope is to create treatments that not only improve quality of life but potentially cure genetic diseases.
Overcoming Challenges in Gene Editing
Despite the tremendous promise of gene editing technologies, several challenges remain. One of the primary hurdles is ensuring the safety and efficacy of these treatments before they can be widely adopted. As David Liu mentions, it is crucial for researchers to conduct thorough testing to minimize the risks associated with these novel therapies. Regulatory considerations are also paramount, as successful application in clinical settings requires rigorous oversight to protect patients and uphold ethical standards.
Moreover, there are inherent societal challenges regarding the perception and acceptance of gene editing. Misunderstandings and ethical dilemmas surrounding genetic manipulation could hinder progress in research and implementation. Engaging with communities and providing clear communication about the benefits and risks involved in gene editing is essential for fostering public trust. As scientists, educators, and policymakers work together to navigate these challenges, the future of gene editing can become a collaborative effort grounded in progress and responsibility.
The Role of Basic Science in Gene Editing
The foundation of gene editing advancements lies in the realm of basic scientific research. David Liu emphasizes that discoveries in basic science often reveal fundamental truths about genetic mechanisms, paving the way for technological innovations. It was thorough investigation into DNA structure and function over decades that led to the momentous discovery of CRISPR technology, demonstrating how curiosity-driven science can yield transformative applications. Without this foundational work, breakthroughs like base and prime editing may not have materialized.
Basic science is vital because it seeks to understand the unknown and pushes the boundaries of knowledge without the immediate pressure of application. This foundational understanding of genetic systems has led to the development of tools that can accurately manipulate the genetic code. Liu’s advocacy for basic research stresses that the pursuit of knowledge for its own sake can lead to the remarkable advances we are seeing in gene editing and its applications in fields ranging from healthcare to biotechnology.
Future Considerations in Genetic Research
As gene editing continues to evolve, future considerations must address both the scientific and ethical dimensions of this powerful technology. Researchers like Liu express concern about maintaining a robust partnership between academia and governmental agencies to nurture the next generation of scientists. The current landscape poses numerous challenges that threaten to stifle innovation and collaboration—dynamics that are essential for breakthroughs in understanding and medical applications of genetic technologies.
The future of genetic research must also engage public dialogue about the implications of gene editing advancements. By fostering open conversations about the benefits and potential risks, scientists can better prepare society for the changes that these technologies will bring. Addressing ethical concerns and ensuring equitable access to gene therapies will be critical as these innovations become more integrated into our healthcare systems. In striving for a responsible and inclusive approach, the benefits of gene editing can extend to all, ultimately shaping a more equitable future.
Frequently Asked Questions
What are the latest advancements in gene editing technologies like CRISPR and base editing?
Recent advancements in gene editing technologies, particularly CRISPR, base editing, and prime editing, have transformed the field of genetics. CRISPR technology continues to be a cornerstone, providing precise cuts in DNA for gene disruption. Base editing, developed by scientists like David Liu, allows for direct single base changes without needing to cut the double helix, making it ideal for correcting mutations that cause genetic diseases. Prime editing takes this further by enabling more complex edits, such as adding, deleting, or replacing DNA sequences, effectively functioning like a word processor for genomic sequences.
How is David Liu’s work with base editing impacting the treatment of genetic diseases?
David Liu’s work on base editing has significant implications for the treatment of genetic diseases. His approach allows for precise corrections of common mutations associated with various conditions by directly altering the DNA bases. This groundbreaking technology has already led to clinical trials where patients with genetic disorders, such as T-cell leukemia, have shown remarkable recovery outcomes after treatment with base editing, showcasing its potential as a powerful tool in modern medicine.
What are the differences between CRISPR, base editing, and prime editing in gene editing?
CRISPR, base editing, and prime editing serve different roles in gene editing. CRISPR technology involves cutting DNA strands to disrupt genes, which can sometimes lead to unintended consequences. Base editing, pioneered by David Liu, modifies DNA bases directly without cutting the DNA, thus minimizing potential off-target effects and allowing for precise corrections of genetic mutations. Prime editing is an even more advanced technique that enables users to search and replace DNA sequences accurately, akin to using a text editor, making it possible to address a broader array of genetic mutations.
What role does base editing play in clinical trials for genetic disease treatment?
Base editing plays a crucial role in clinical trials aiming to treat genetic diseases. As of now, numerous clinical trials are utilizing base editing to address various conditions. Notably, some patients treated with base editing technologies have reported significant improvements in their health, leading to a reduction in symptoms and the need for medications. This innovative approach is reshaping our understanding of potential therapies for previously untreatable genetic conditions.
How has CRISPR technology paved the way for advancements in genetic disease treatment?
CRISPR technology has been foundational in advancing the treatment of genetic diseases. Its discovery ignited research into gene editing, leading to the development of more refined techniques like base editing and prime editing. By enabling targeted genetic modifications, CRISPR has empowered scientists to explore therapeutic interventions for a wide array of genetic disorders, establishing a platform from which novel treatments are continually emerging.
What are the future prospects of gene editing advancements in healthcare?
The future prospects of gene editing advancements in healthcare are incredibly promising. With ongoing research and breakthroughs in technologies like CRISPR, base editing, and prime editing, the potential to effectively treat and even cure genetic diseases is growing. As these techniques advance, we can expect more clinical applications in personalized medicine, providing tailored treatments that address the genetic underpinnings of various ailments, thus revolutionizing healthcare delivery.
| Key Points | Details |
|---|---|
| Novel Gene Editing Technologies | Base editing and prime editing are innovative methods developed to correct genetic diseases by targeting single nucleotide changes. |
| Success Story: Alyssa Tapley | At age 13, Tapley’s T-cell leukemia was treated successfully with base editing technology in a clinical trial, marking a significant scientific breakthrough. |
| The Role of David Liu | David Liu, a leading scientist, emphasizes the ethical responsibility in ensuring the safety and efficacy of gene-editing techniques. |
| Historical Significance of CRISPR | The journey of CRISPR’s discovery from basic science in E. coli to its application in gene editing illustrates the importance of fundamental research. |
| Clinical Trials | As of 2025, there are at least 18 clinical trials exploring base and prime editing for genetic diseases, demonstrating real-world applications. |
Summary
Gene editing advancements have significantly changed the landscape of medical treatment, particularly for genetic diseases. The development of base editing and prime editing technologies provides a new pathway to correct genetic mutations at a molecular level. With successful cases like Alyssa Tapley’s, these innovations demonstrate the potential to transform patient outcomes and offer hope to millions suffering from genetic disorders.